Alnylam Pharmaceuticals


ABOUT ALNYLAM
Our Science Is Changing the Way Medicine Treats DiseaseTM
Alnylam has led the translation of RNAi (RNA interference) from Nobel Prize-winning discovery into an innovative, entirely new class of medicines. Founded in 2002 by a team of distinguished life sciences leaders, Alnylam’s vision is to harness the potential of RNAi therapeutics to transform the lives of people living with diseases for which there are limited or inadequate treatment options. Our pioneering work has delivered the world’s first and only approved RNAi therapeutics—ONPATTRO® (patisiran) in 2018, GIVLAARI® (givosiran) in 2019, OXLUMO® (lumasiran) in 2020, and AMVUTTRA® (vutrisiran) in 2022. We are advancing a deep pipeline of innovative RNAi-based medicines in four therapeutic areas: genetic medicines, cardio-metabolic diseases, infectious diseases, and central nervous system (CNS) and ocular diseases.

What’s in a Name?
AL-NY-LAM. Our name may not be the easiest to pronounce, but once you learn it, you’ll never forget it. Alnylam is derived from “Alnilam,” the bright center star in the constellation Orion’s belt, which has been used by navigators for thousands of years and symbolizes our passion for discovery.
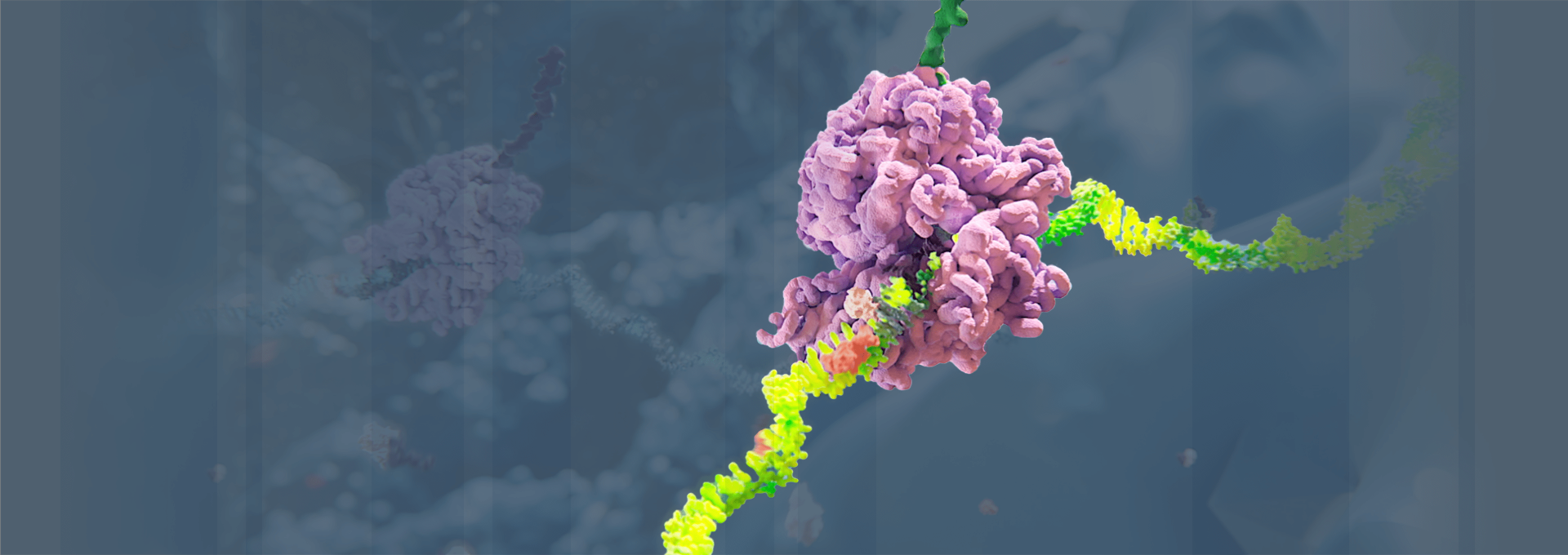
RNAi Therapeutics—An Innovative New Class of Medicines
Medicines based on RNAi work by “silencing” or disabling the production (“expression”) of the genes that cause specific diseases. In doing so, RNAi therapeutics work “upstream” of most other classes of medicines, such as small molecules and monoclonal antibodies, by targeting the “root” genetic cause of a disease rather than its symptoms. To learn more about our science, click here.
RNAi is the core discovery that forms the therapies Alnylam is developing. It is recognized as a major scientific breakthrough—but how does it work, exactly?
Vision, Mission, and Values
Our Vision - Harnessing a revolution in biology for human health®.
Our Mission - Build a top-tier, global, independent biopharmaceutical company founded on RNAi.
Our Core Values - Commitment to People, Innovation & Discovery, Sense of Urgency, Open Culture, and Passion for Excellence.
CAREERS AT ALNYLAM
Join Our Team and Grow With Us
At Alnylam, we are driven by our mission to translate the Nobel Prize-winning discovery of RNA interference (RNAi) into an innovative, new class of medicines. We’re motivated in this endeavor by the bravery and perseverance of people with unmet medical needs who live with rare and genetic diseases. They are the focus of our efforts, and we exist as a company because of them. That knowledge and commitment to patients is present in everything we do, every day.

At The Forefront of What’s Next
For Patients
Our goal, which is well underway, is to become a world-class biopharmaceutical company that can independently develop and deliver innovative medicines based on RNAi to patients worldwide. We have a deep pipeline of early to late stage investigational therapies, and 2018 marked the approval of our first medicine—the world’s first RNAi therapeutic. Even though we’ve been around since 2002, we’re just getting started!
For Employees
We are growing quickly and hiring across North America, Europe, Asia, and Latin America for a wide variety of roles. We’re seeking smart, passionate, “change the world” kind of people who are ready to say, “challenge accepted.” Our 1,500 (and counting!) employees are building a diverse, equitable, inclusive, and high-performing global organization that celebrates team achievement and recognizes individual contributions.
We’re at the forefront of what’s next for patients, and perhaps your career also.
Search among Alnylam Pharmaceuticals jobs
| Jobs: 71 - 80 of 95 |

Senior Scientist, Analytical Sciences
Cambridge, Massachusetts
Overview: Analytical Development at Alnylam is seeking a highly motivated Senior Scientist with expertise in Bioassay including cell-based assays using different cell types and detection formats, qPCR, ELISA . This role involves performing ...
17d
| Job Type | Full Time |

Cambridge, Massachusetts
Overview: The Senior Statistician will be a member of the Statistics team in the Chemistry, Manufacturing, and Controls (CMC) department. The Senior Statistician will apply statistical techniques in partnership with multidisciplinary teams ...
17d
| Job Type | Full Time |

Regional Business Director - TTR (South/Central Florida - Miami, Ft. Lauderdale, Tampa)
Cambridge, Massachusetts
Overview We are looking for an experienced Regional Business Director (RBDs to support the promotion of AMVUTTRA (vutrisiran). The U.S. Food and Drug Administration (FDA) recently approved the supplemental New Drug Application (sNDA) for AM...
17d
| Job Type | Full Time |

Senior Medical Director, Cardiology
Cambridge, Massachusetts
Overview The Senior Director, Cardiology serves as the U.S. medical and scientific subject matter expert (SME) for ATTR amyloidosis and related cardiology disease states. This highly visible role blends scientific expertise, external leader...
17d
| Job Type | Full Time |

Manager, Manufacturing Systems
Cambridge, Massachusetts
Overview The Manager, Manufacturing Systems is an integral part of the Internal Manufacturing team. This role is the end-user subject matter expert for the Manufacturing Execution System (MES) and Real-Time Modeling System (RTMS), The SME w...
18d
| Job Type | Full Time |

Cambridge, Massachusetts
Legal Director, R&D and Regulatory The Legal Director, R&D and Regulatory will serve as a trusted advisor providing expert legal counsel and strategic guidance across Alnylam's Research & Early Development (ReDev), Regulatory, Global Patien...
18d
| Job Type | Full Time |

Principal Scientist, Advanced In Vitro Platforms
Cambridge, Massachusetts
Overview We are seeking an accomplished and motivated Principal Scientist to lead innovation in advanced in vitro modeling to enable RNAi discovery and development. In this role, you will be responsible for designing, validating, and implem...
18d
| Job Type | Full Time |

Cambridge, Massachusetts
Alnylam is the world's leading RNA interference (RNAi) company, dedicated to transforming the lives of patients through innovative medicines. As we continue to expand our global footprint and digital presence, the role of enterprise web pla...
18d
| Job Type | Full Time |

Principal Scientist, Antibody Cell Line Development
Cambridge, Massachusetts
Overview: We are seeking a Principal Scientist to join the Protein Sciences team to drive cell line and upstream process development for the production of monoclonal antibodies and antibody-oligo conjugates. The candidate will work across f...
18d
| Job Type | Full Time |

Scientist, Analytical Sciences
Cambridge, Massachusetts
Overview: The successful candidate will be responsible for supporting clinical-stage programs within Analytical Sciences at Alnylam Pharmaceuticals. Responsibilities will include the development, qualification, and transfer of analytical as...
18d
| Job Type | Full Time |

