Agendia

Dedicated to pristine science in personalized medicine.
Agendia is the only molecular diagnostics company focused solely on breast cancer. With our groundbreaking genomic testing, we generate reliable and meaningful clinical data about the unique biology of a woman’s breast cancer. Backed by prestigious physicians and institutions globally, we are shifting the standard of care towards more ideal, personalized treatment.
OUR MISSION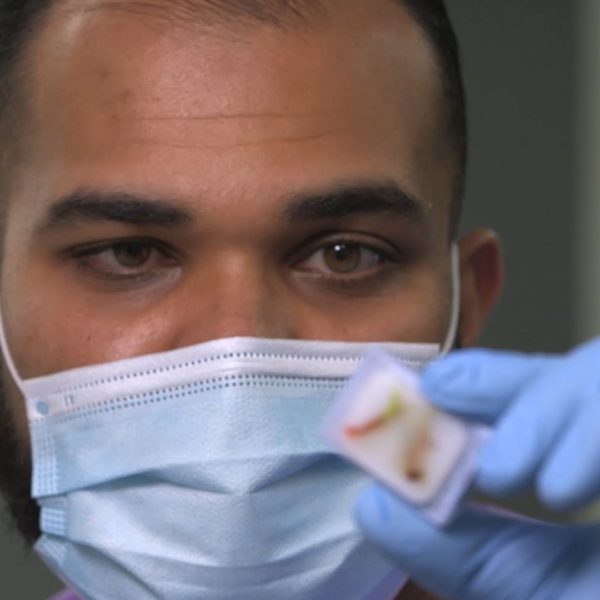 Providing clarity and confidence on the path to recovery.
Providing clarity and confidence on the path to recovery.
We believe in the power of genomic testing to better inform the patient journey – from the moment of diagnosis to remission, and beyond. It sheds light on complex treatment decisions, leading to the most relevant and targeted approach for each individual patient.
In helping doctors achieve the best possible outcomes, we hope to improve their patients’ overall quality of life.
Careers
Let’s take on cancer together.
You are exceptionally talented. You see no greater mission than improving the lives of women with breast cancer. And you understand the importance of addressing their unique needs on the journey to recovery.
Join other like-minded innovators and out-of-the-box thinkers in our pursuit of meaningful change in breast cancer care.
OUR CULTURE
Community. Curiosity. Creativity.
We pride ourselves on creating an inclusive and motivational atmosphere. Our passion for doing good drives us to continue innovating and tapping all the resources around us in that process. We value the diverse perspectives of our team and take measures to make each member feel heard.
Guided by Five Principles
 Integrity: Consciously and intentionally making decisions that reflect Agendia’s commitment to ethical, honest and authentic answers for patients.
Integrity: Consciously and intentionally making decisions that reflect Agendia’s commitment to ethical, honest and authentic answers for patients.- Clarity: Providing certain and transparent actions and communication understanding that clear communication affects a patient’s experience.
- Accessibility: Purposefully seeking ways to break down barriers that impede communication and embracing alternate perspectives which may result in ground-breaking ideas.
- Responsiveness: Promptly responding to request and issue in a calm and thoughtful manner, providing the timeliness needed for important decisions.
- Excellence: Striving to go above and beyond in our work, knowing that our actions impact the life of a patient with breast cancer.
Our reputation says it all.
We changed the game when we commercialized our two best-in-class genomic tests, MammaPrint and BluePrint. They continue to provide physicians with vital information that can guide the course of a woman’s breast cancer treatment – ultimately, impacting her life and all those around her for the better.
Many of the world’s top medical minds trust in our science and the pristine data that supports it. We entreat you to trust in us as well – to work alongside respected thought leaders and scientists to further advance precision medicine.
Search among Agendia jobs
| Jobs: 1 - 3 of 3 |
Molecular Oncology Specialist (Sales)
Boston, Massachusetts
Apply Description **Starting at $250k inclusive of Base Salary + Comm AIM OF THE POSITION The Molecular Oncology Specialist's (MOS) primary responsibilities are to develop and successfully execute sales strategies to ensure achievement of r...
6d
| Job Type | Full Time |
Irvine, California
Apply Description AIM OF THE POSITION The purpose for this position is for an individual to prepare, process, maintain, and manage all aspects related to Billing including benefit and eligibility verification, Order Entry, QC, primary billi...
18d
| Job Type | Temporary |
Prior Authorization Coordinator
Irvine, California
Apply Description AIM OF THE POSITION The Prior Authorization Coordinator is responsible for requesting authorization for all applicable incoming orders. This process includes understanding of payers' medical policies for our services, coll...
24d
| Job Type | Full Time |

